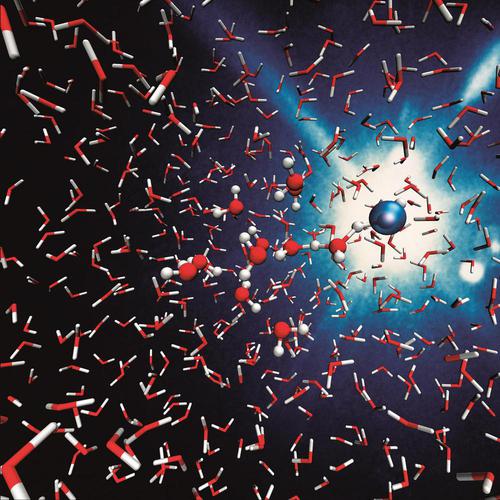
Real-Time Chemical Dynamics
Liquid water is a ubiquitous solvent and, as such, is a ubiquitous medium for chemical reactions. The elementary steps underlying these reactions take place on the time scale of femtoseconds, whereas the reaction kinetics can unfold on time scales that extend up to minutes. In order to capture and characterize these processes, pillar 4 focus on the real-time chemical dynamics in liquid water. One of the central questions that we address is how the ultrafast structural response of water molecules affects the energetics—and, thus, steers the pathways—of chemical reactions in nuclear configuration space. Another key challenge is the identification of the ultrafast mechanisms of reactive processes connected to proton, electron, and OH-transfer, which form the basis of acid-base and redox chemistry in liquid water. A third example is water radiolysis, which plays a central role in, e.g., radiotherapy and gives rise to the formation of transient, highly reactive radicals such as H2O+andOH. Particularly the former species is extremely challenging to detect because of its high reactivity in water.
We address these and related challenges by combining experimental techniques such as state-of-the-art photoelectron spectroscopy and ultrafast laser technology with theoretical methodology such as first-principles non-adiabatic molecular-dynamics simulations. In addition to real-time chemical-reaction dynamics in homogenous aqueous solutions, we will employ time-resolved methods to develop an understanding of reaction dynamics at aqueous interfaces.
Speakers: Francesca Calegari (DESY/U Hamburg), Uwe Hergenhahn (IOM), Robin Santra (DESY/U Hamburg), Iain Wilkinson (HZB).